Battery Questions, Simplified Answers
What exactly is happening inside a battery when it’s getting used?
The specifics can vary among battery types, but the core idea remains the same: two different metals interact with a liquid that wants to corrode them both. These corrosion reactions are cleverly designed so that one metal ends up with too many electrons, while the other has too few. This creates a voltage difference between the two. Eventually, the reactions hit a limit where the chemical energy can’t shift more electrons, and the system stalls. Connecting the metals allows electrons to flow, restarting the reactions.
The process continues until the battery exhausts its reactive materials. Reversing the flow of electricity can undo these reactions, restoring the original materials. However, this requires careful design to manage the reactions and avoid gas buildup, which could cause the battery to explode. High-quality rechargeable batteries are engineered to handle this safely, allowing for hundreds of charge cycles. That said, this often means they store slightly less energy overall.

Why are there different sized batteries (aaa-D, etc) if they all put out the same 1.5v?
The size of the battery changes the capacity (how much total power is stored) rather than voltage. A large D-cell battery will run longer than a AA-cell for the same power draw.
The voltage is dictated by the chemistry inside of the battery. Basically, different elements or molecules have different electro-chemical potentials. That’s why alkaline, lead-acid, NiMH, Li-Ion, LiFePO4, etc all have different voltages: they all have different chemistries.
You have different size batteries for different uses that may have other design choices. For instance, you don’t want to use giant D-cell batteries for your Tv remote control because that would be uncomfortable in the hand and remotes use very little power. However, if you have something larger that has heavy power draw, maybe those D-cell batteries make more sense.

Why are AA and AAA batteries shaped like cylinders?
cylindrical shape of many common batteries is that they allow for equal internal pressure around the exterior, which makes them less likely to rupture and cause injury or damage. It’s the same reason you see cylindrical designs in things like SCUBA tanks or propane tanks or those tanker trucks that deliver gasoline to your local station.
When making a battery, you want it to be as efficient with space as possible, while still making it easy to design. Batteries with sharp corners or edges would be very hard to make, and would be less space-efficient.
Battery design is primarily about getting the best battery performance, not about making the batteries fit nicely in something. I think most folks would accept an awkward fit if it meant the battery lasted twice as long (or whatever.)
How are batteries made?
- Think of making a battery like building a layered cake. You start with the “layers,” which are electrodes—a positive side (cathode) and a negative side (anode). These are coated with active materials, much like spreading frosting on a cake. Between these layers, a separator is added, like a thin sheet of wax paper, to keep them from touching while allowing ions to flow. Everything is then wrapped tightly and filled with an “electrolyte” (think of it as the sauce that makes everything work together). Finally, the whole package is sealed into a case to keep it safe and ready to deliver energy.
What is battery cycles?
If you’ve ever bought a used laptop or phone, you’ve likely come across the term “Cycle Count.” A battery cycle refers to one full charge and discharge process. For example, if you charge a battery from 50% to 100% and then use it back down to 50%, that counts as half a cycle. Battery cycles are a key indicator of a battery’s health and longevity, as most batteries are rated for a specific number of cycles before their capacity significantly diminishes. This count helps users estimate how much life remains in the battery and is especially important in evaluating used devices.
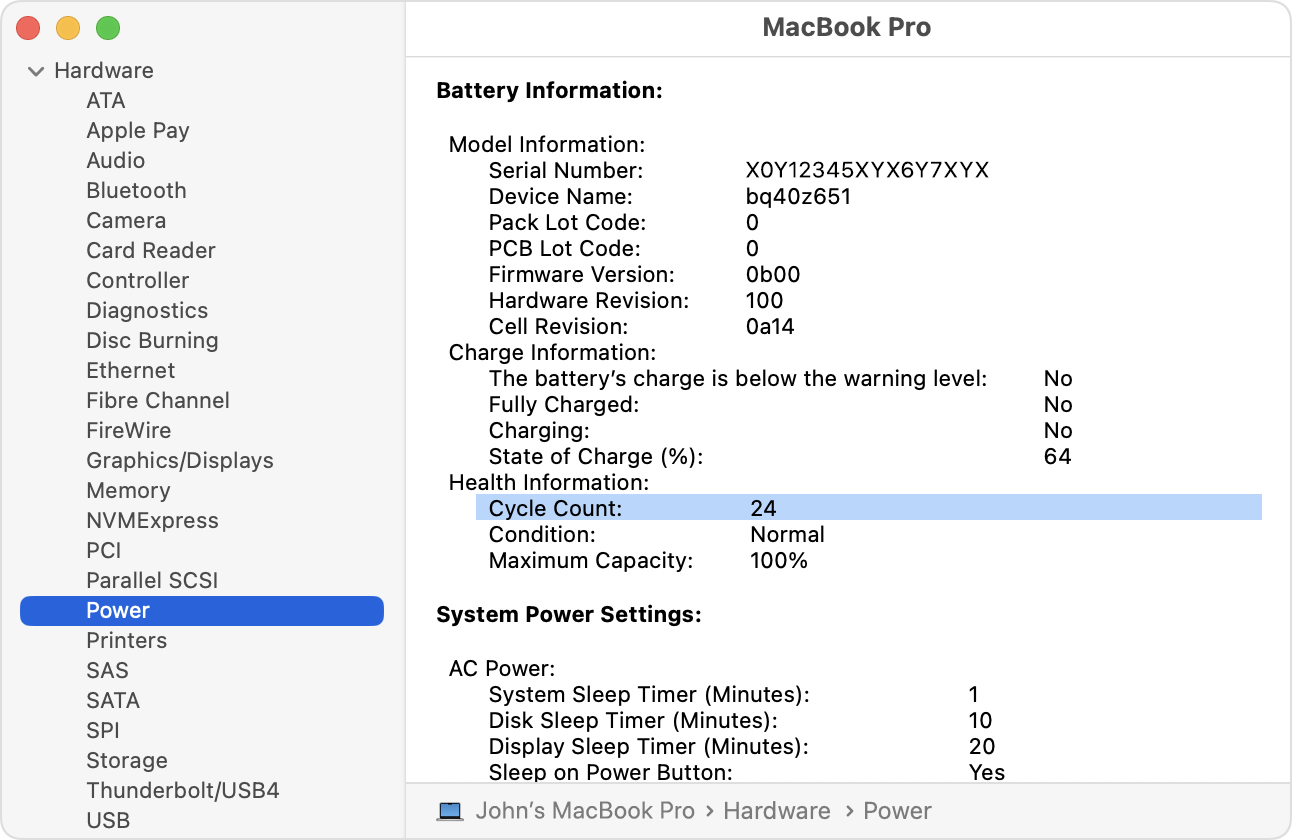
How many charge-discharge cycles can a battery handle ?
- The number of cycles a battery can handle depends on its chemistry and usage. For example, lithium-ion batteries typically last between 300 to 500 cycles, while high-quality lithium iron phosphate (LiFePO4) batteries can exceed 2,000 cycles. Factors such as charging speed, depth of discharge, and operating temperature also play a significant role in determining a battery’s cycle life. Understanding this helps users maximize the lifespan of their batteries through optimal usage and maintenance.

Why do batteries degrade over time?
Batteries have a few main parts: the anode (negative), the cathode (positive), a separator between them, and some stuff in between (usually a liquid) that conducts ions. When you charge a battery, you are cramming a whole bunch of lithium ions into the anode, kind of like absorbing water into a sponge. When you use the battery, these ions flow to the cathode, generating electric current. Over time, by cramming the ions in and out of the anode and cathode, you begin to damage the ‘sponge’, so it can’t hold as many ions anymore. So your efficiency goes down.
Most traditional batteries use a combination of an anode and cathode material, often lithium cobalt oxide for the cathode and graphite for the anode. During charging and discharging, lithium ions move back and forth between these electrodes, creating the flow of electricity. However, some lithium ions irreversibly react with other components in the battery, becoming trapped and unable to cycle. Over time, this reduces the battery’s capacity. Other issues include the growth of lithium dendrites or physical stresses on the electrodes, which can further degrade performance. Additionally, repeated ion movement can create stress fractures in the anode and cathode materials, similar to how bending a paperclip repeatedly weakens it. This compromises the structural integrity of the battery, leading to reduced performance and eventual failure.
What happens to a battery during a charge-discharge cycle?
Imagine your battery as a courier system. Charging is like loading up the van with packages (lithium ions) and moving them to one side of the battery (the anode). Discharging is delivering those packages to customers (the cathode) by releasing energy to power your device. Over time, the courier van (the battery) gets a little worn out: some packages (ions) get stuck, and the van’s parts (the electrodes) degrade. This wear and tear with every trip (charge-discharge cycle) means the battery can carry fewer packages, holding less energy as it ages.
Should you charge a battery to 100% before using it for the first time?
You’re thinking of the memory effect from old Nickel Cadium (NiCad) or Nickel-metal Hydride (NiMH) batteries, but not with Lithium batteries that we’re now using. These don’t have a “memory effect” to this extent, and in fact fully charging or discharging them is what’s worst for the battery. It’s better to always keep them in the 10%-90% range, and some smartphones even do that themselves.
It can still be a good idea to do one full charge and one full discharge on new devices - but not for the battery itself, just so the battery management systems learns the capacity of the battery and the phone can accurately tell you how full the battery is, but this process is done pre-sale in the factory these days
Why is it better to keep batteries in the 10%-90% range ?
Lithium-ion batteries work by moving atoms (like lithium ions) back and forth between two sides of the battery. These atoms are absorbed into solid particles on each side, which are made of materials like powdered graphite (similar to pencil lead). These particles act like a scaffolding: they allow a certain number of atoms to move in and out without breaking their structure.
You can think of this scaffolding as flexible but fragile: Too many atoms removed or added (over-draining or over-charging): The structure collapses. Once this happens, the atoms get “stuck,” and they can’t move back and forth as easily, permanently reducing the battery’s capacity.
Battery manufacturers are aware of this risk, that why when your phone says “100%” charged, it’s often only at 90% of its true capacity to prevent over-charging and when your phone “dies,” it still has around 10-15% charge left as a buffer to avoid over-draining.
Why does charging a battery’s last 10% take longer than the rest?
Think of it like parking cars in a crowded lot:
Early on: There are plenty of spaces, so you can park quickly.
Near the end: Finding the remaining open spots requires more precision and takes more time.
Similarly, as the battery nears full, the charger reduces the current to avoid overheating or overcharging. This slower “trickle charging” protects the battery and ensures its safety and longevity.

How do electronics measure and display battery percentage ?
Voltage isn’t exactly constant. A cell phone battery might be rated at 3.7 volts, but really it’s 3.8V when it’s fully charged, and 3.5V when it’s empty. The phone then has (more or less) a look-up table. The phone knows that when it’s at 3.8V, it’s 100% charged. And when it’s at 3.75V it’s 80% charged, etc. This is also why old phones sometimes go from 20% charged to 0% charged almost instantly - because the battery is old and isn’t performing according to the lookup table. this way is called Voltage Translation and it’s one of many ways
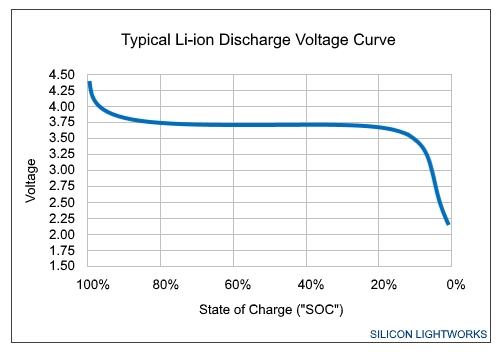
another way is Coulomb Counter: coulomb counter looks at current (amps) per second. Some systems actually do look at voltage too, but cheaper chips for cell phones won’t. Voltage is usually only used to trigger battery warnings and not used for fuel gauging since it is so flat during discharge. Especially if the system designer only wants the battery to stay in that flatter near constant region of the lithium battery curve.
one other way is fuel gauge. (This is the best method for measuring li-po batteries because the voltage varies only a little with state of charge and will change a lot depending on the draw from the battery and its age. Alternative battery chemistries have much more linear changes in voltage, so getting state of charge from voltage works fine for those.) If I give you $100, you will know you now have $100 in your pocket. Your pocket just got charged up. As you spend it you keep track of how much you’ve spent and you will know therefore how much you have left.The faster you spend it (higher power) the faster you run out of money (energy). You can add or subtract from your total as you go and as long as you keep track, you’ll know how much you have left.
Why does my car battery seem fine until I try to start the engine?
With car batteries (the 12V lead acid type) the voltage isn’t really a good indicator of health. An old dead battery can read ~12V just fine. It would likely power most lights and equipment, too. The real test of health comes when trying to start the engine; the “load” test. An old battery can read 12V until asked to turn the starter, then immediately drops to an unusable voltage.
The simple answer is that traditional 12V car batteries do not have the sophisticated tech to indicate their health like, say, laptop batteries. Nor is there a good way to test the health except for hooking the battery to a load, which isn’t an easy thing to build into a car’s circuitry. Basically, starting the engine is the load test.

Does a drained battery weigh less than a fully charged one
- Technically, according to Einstein’s E=mc², the energy stored in a fully charged battery could theoretically add an imperceptibly tiny mass to the battery. However, the effect is so small (on the order of nanograms or less) that it’s impossible to measure with current tools. It’s purely a theoretical concept and not practically noticeable.
How does Fast-charging work ?
Fast charging works by increasing the amount of current delivered to the battery during charging. Normally, a standard charger provides a low, steady current to gradually fill up the battery. A fast charger, on the other hand, pushes more current into the battery in a shorter time. This is made possible by advanced technologies like voltage regulators, temperature monitors, and smart circuits that manage how much current the battery can safely accept without overheating.
To ensure safety, fast-charging systems are equipped with heat sensors and software that adjust the charging rate dynamically. If the battery gets too warm, the system slows down the charge to prevent damage or thermal runaway (a dangerous condition where heat builds uncontrollably). Modern batteries are also designed with internal safeguards, such as pressure-sensitive vents and separators, to minimize risks.

Why fast chargers might not be good for your battery?
Fast charging, while convenient, can take a toll on your battery over time. The process involves pushing a lot of current into the battery, which generates heat and can lead to lithium plating. This occurs when lithium ions form a coating on the anode instead of embedding properly, as illustrated in the image. Over time, this reduces the battery’s capacity and causes internal wear. You can see in the diagram how lithium plating creates high-resistance regions and excess lithium areas, both of which degrade the battery’s performance and longevity.
Thankfully, modern devices include features like temperature control and tapering, which slow charging as the battery fills to prevent overheating.
Why can’t we just instantly-charge our batteries?
One of the main challenges with charging batteries quickly is managing heat. Overcharging generates excess heat, which can lead to dangerous situations like fires or explosions. In response to this issue, companies like NIO in China have introduced battery swapping stations. Instead of waiting for a battery to charge, the depleted battery is replaced with a fully charged one, bypassing the heat problem entirely.
